ENERGY BANDS IN SOLIDS
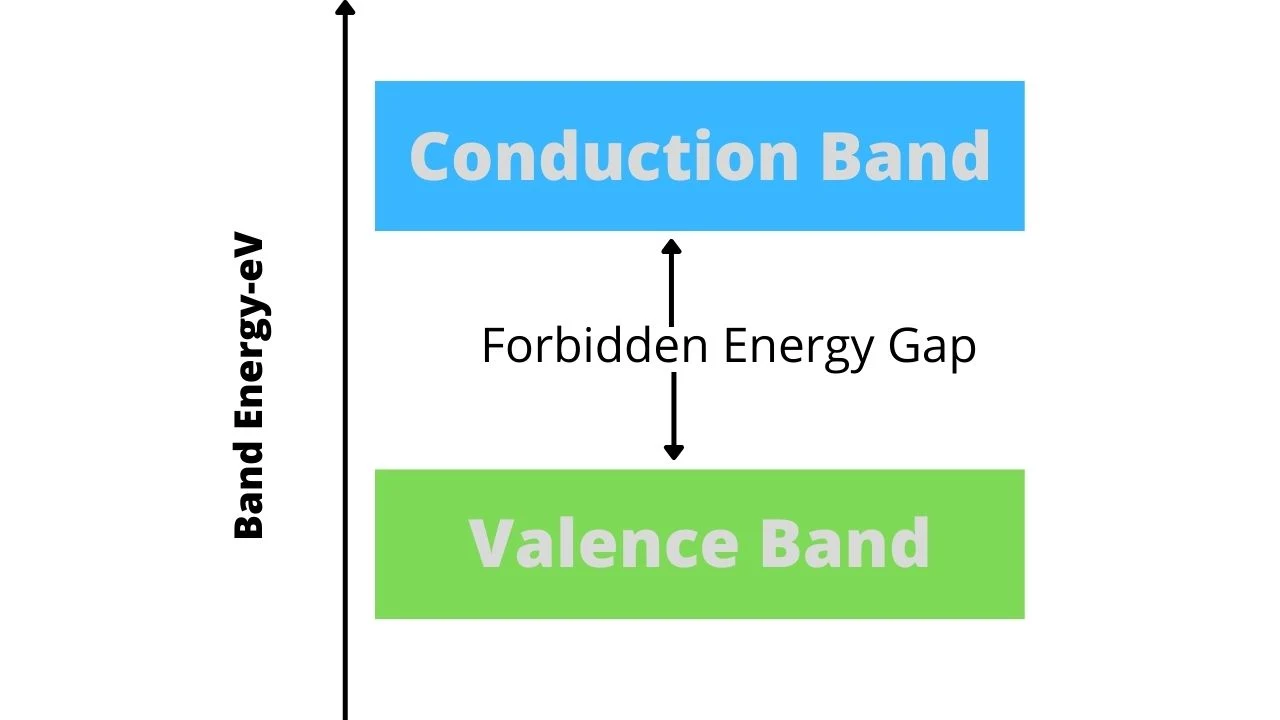
1. Valence Band:
The electrons in the outermost shell of an atom are called valence electrons. They have the least binding energy, though their orbital energy is maximum. The energy band occupied by the valence electrons is called the valence band. It is the highest occupied band. In the case of inert gases, the valence band is full, whereas in other materials it is only partially filled but can never be empty.
2. Conduction Band:
In certain materíals, the valence electrons are loosely attached to the nucleus. Even at ordinary temperature, some of the valence electrons may get detached to become free electrons. These free electrons are responsible for the conduction of current. For this reason, these electrons are called conduction electrons: The band possessed by conduction electrons is known as the conduction band. It may either be empty or partially filled. It may be defined as the lowest unfilled energy band in an atom.
3. Forbidden Energy Gap :
The separation between the conduction band and valence band in the energy level diagram is known as the energy gap. No electrons of a solid can stay in the forbidden energy gap as there is no allowed energy state in this region. The width of this gap is a measure of the bondage of valance electrons to the atom. The greater the energy gap more tightly the valence electrons are bound to the nucleus. In order to push an electron from the valence band to the conduction band external energy equal to the forbidden energy gap must be supplied.